FDA Authorizes Pfizer Jabs For Children Between Ages 5 And 11
After CDC and FDA Advisory panels debated the to the safety of vaccinating younger, less vulnerable, COVID patients who might be at a higher risk of heart inflammation from COVID jabs, the Biden Administration has once again struck a compromise between “the science” and personal freedom.
On Friday, the FDA has decided that the Pfizer-BioNTech jab is safe for children between the age of 5-11, with doses spread three weeks apart, and using a smaller formulation of the vaccine that includes only 10 micrograms, as recommended earlier this week by the FDA in a unanimous vote.
After CDC and FDA Advisory panels debated the to the safety of vaccinating younger, less vulnerable, COVID patients who might be at a higher risk of heart inflammation from COVID jabs, the Biden Administration has once again struck a compromise between “the science” and personal freedom.
Here are some key points from the FDA for parents and caregivers, starting with the fact that children ages 5 to 11 aren’t really any more or less infectious than those up to the age of 25.
In the study, the vaccine was 90.7% effective in preventing COVID-19 in children 5 through 11, which is roughly the same level of protection that the Pfizer vaccine vaccine purportedly provided to older patients.
Safety: The vaccine’s safety was studied in approximately 3,100 children age 5 through 11 who received the vaccine and no serious side effects have been detected in the ongoing study.
Dr. Janet Woodcock, an MD and the acting FDA commissioner, said she knows how long many parents have been desiring this latest data so they can get their children vaccinated as soon as possible. Some school districts in LA and NYC have demanding that vaccines be used.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” said Acting FDA Commissioner Janet Woodcock, M.D. “Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards.”
The Pfizer-BioNTech COVID-19 vaccine for children 5 through 11 years of age will be administered as a two-dose primary series, with doses stretched 3 weeks apart as mentioned. However, unlike the prior adult jabs, it’s a lower dose (10 micrograms) than that would be used for individuals 12 years of age and older (30 micrograms) so as to lower the risk of any unintended side effects.
The Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices will meet next week to discuss further clinical recommendations.
The biggest change in the FDA’s authorization for the child brand is a formulation that uses a different buffer; buffers help maintain a vaccine’s pH (a measure of how acidic or alkaline a solution is) and stability. This new formulation is more stable at refrigerated temperatures for longer periods of time, permitting greater flexibility for vaccination providers.
For now at least, COVID cases in the US have continued to shrink. But Scientists are keeping an ever vigilent eye on the situation
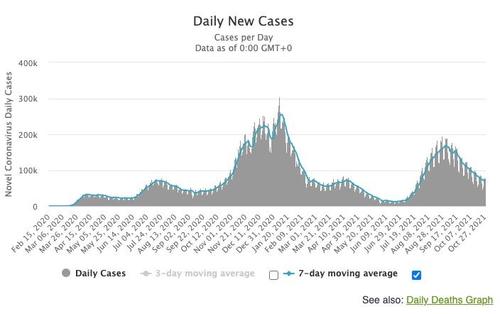
The US isn’t the only country allowing vaccines for the very young, typically seen as among the least vulnerable. As we noted the other day, some countries, including China, have already started vaccinating people as young as 3.
Per the FDA’s statement, The authorization was based on the FDA’s “thorough and transparent” evaluation of the data that included input from independent advisory committee members who voted overwhelmingly to approve the jab for children as young as 5.
Now that the FDA has approved jabs for those between the ages of 5 and 11, their goal will shift to winning authorizing even young patients, potentially as young as 6 months old.